Phytochemical Profiling of Methanolic and Ethanolic Extracts of Pemphis acidula (J.R.Forst. & G.Forst.) Leaf using LC–MS/MS and GC–MS
Downloads
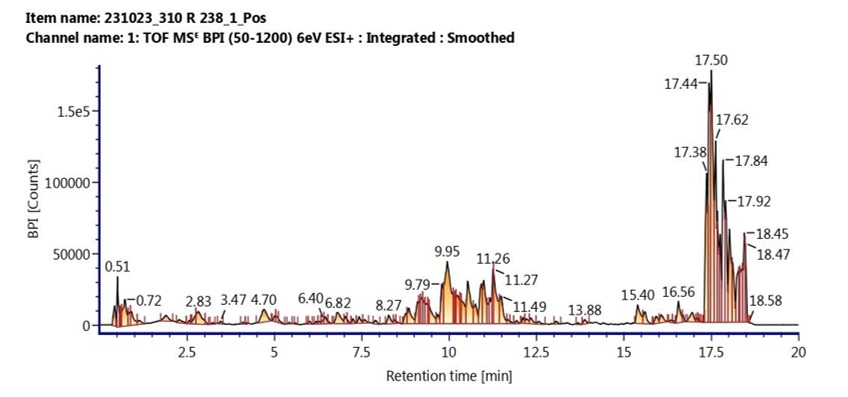
Pemphis acidula (J.R.Forst. & G.Forst.) is a mangrove species of the Lythraceae family widely distributed along the Indonesian coasts. Traditionally used for medicinal and cosmetic purposes, information on its phytochemical composition remains limited. Comprehensive chemical profiling is essential to elucidate its bioactive potential and development. This study aimed to identify, characterize, and compare the phytochemical and bioactive profiles of methanolic and ethanolic extracts of Pemphis acidula using LC–MS/MS and GC–MS to determine which solvent yields richer bioactive composition. Leaf samples were collected and authenticated at Herbarium Bogoriense (BRIN). The dried powders were extracted with methanol or ethanol by triple maceration for 24 hours, and filtrates were evaporated under reduced pressure. Crude extracts were analyzed using LC–MS/MS (UPLC-QTOF, ESI ±) and GC–MS; compound identification employed UNIFI software referencing the Waters Traditional Medicine Library and Wiley 275 database. The results showed that LC–MS/MS identified 25 compounds in methanolic and 49 in ethanolic extracts, while GC–MS detected 13 and 19 compounds, respectively, with quality matches ≥90%. These compounds comprised alkaloids, flavonoids, phenols, polyphenols, and terpenoids with antioxidant, anti-inflammatory, antibacterial, antiviral, and anticancer activities. Eleven non-volatile compounds were identified in both extracts, namely kaempferol-3-glucuronide, desmanthin, quercetin-3-O-α-D-glucuronide, quercetin-3-O-glucuronide 6″-methylester, ellagic acid, Z-ligustilide, melazolide A, cimicifugic acid B, vellerdiol, 5,6,7,7α-tetrahydro-4,4,7α-trimethyl-2(4H)-benzofuranone, and farnesyl acetate and seven volatile compounds were identified in both extracts, i.e. supraene, hexadecanoic acid methyl ester, loliolide, 9,12-octadecadienoic acid (Z,Z)-methyl ester, phytol, 9-octadecenoic acid, and tocopherol. In conclusion, Pemphis acidula extracts contain bioactive compounds with medicinal potential, and ethanol extract yielded a broader phytochemical profile than methanol’s.
Downloads
Trifani R, Rabinowitz O, Abdillah S, Sinaga E. GC–MS and LC–MS/MS analysis of Bouea macrophylla fruit juice. International Journal of Biological, Physical and Chemical Studies. 2022;4(2):1–10. https://doi.org/10.32996/ijbpcs.2022.4.2.1
Soraya S, Sukara E, Sinaga E. Identification of chemical compounds in Ziziphus mauritiana fruit juice by GC–MS and LC–MS/MS analysis. International Journal of Biological, Physical and Chemical Studies. 2022;4(2) :11–19. doi: 10.32996/ijbpcs.2022.4.2.2.
Siregar AR, Soraya S, Sinaga E. GC–MS and LC–MS/MS analysis of secondary metabolites in the methanolic extract of Uncaria callophylla Blume ex Korth. stems. International Journal of Biological, Physical and Chemical Studies. 2023;5(2):1–10. https://doi.org/10.32996/ijbpcs.2023.5.2.1
Kristiana L, Paramita A, Maryani H, Andarwati P. Eksplorasi tumbuhan obat Indonesia untuk kebugaran: analisis data riset tumbuhan obat dan jamu tahun 2012, 2015, dan 2017. Jurnal Kefarmasian Indonesia. 2022;12(1):79–89. https://doi.org/10.22435/jki.v0i0.5209
de Wilde WJJO, Duyfjes BEE. Flora Malesiana. Djakarta: Noordhoff-Kolff N.V.; 2016.
Utina R, Katili AS, Lapolo N, Dangkua T. The composition of mangrove species in coastal area of Banggai district, Central Sulawesi, Indonesia. Biodiversitas Journal of Biological Diversity. 2019;20(3):840–846. https://doi.org/10.13057/biodiv/d200330
Baderan Wahyuni K, Baderan DW, Utina R. Biodiversitas flora dan fauna pantai Biluhu Timur (suatu tinjauan ekologi-lingkungan pantai). Deepublish; 2021 Feb 20.
Baderan DWK, Retnowati Y, Utina R. Conservation threats of Pemphis acidula in the Tomini Bay area, Gorontalo, Indonesia. IOP Conference Series: Earth and Environmental Science. 2022;976(1): 012058. https://doi.org/10.1088/1755-1315/976/1/012058
Baderan DWK, Rahim S, Angio MH, Akbar MN, Jannah M, Retnowati Y, Utina R. Diversity of santigi (Pemphis acidula J.R.Forst. & G.Forst.), a mangrove association in Tomini Bay, Sulawesi, Indonesia. Journal of Tropical Biodiversity and Biotechnology. 2024;9(1): jtbb83889. https://doi.org/10.22146/jtbb.83889
POWO (Plants of the World Online). Pemphis acidula J.R.Forst. & G.Forst. 2023. Available from: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:25503-1#source-KBD.
Al Idrus A, Mertha IG, Marhus M, Husain P. Characteristics of Sentigi (Pemphis acidula) as environmental bioindicators of mangrove conservation in the Regional Marine Conservation Area Gili Sulat, East Lombok, Indonesia. Jurnal Penelitian Pendidikan IPA. 2023;9(1):542–549. https://doi.org/10.29303/jppipa.v9i1.2521
Jannah M, Baderan DWK, Rahim S, Angio MH. Genetic variation of Santigi (Pemphis acidula J.R.Forst. & G.Forst.) based on ISSR markers. Proceedings of the 2nd International Conference on Sciences, Mathematics, and Education 2023 (ICOSMED 2023). 2025;927:199–207. https://doi.org/10.2991/978-2-38476-410-5_19
Bourdy G, Walter A. Maternity and medicinal plants in Vanuatu I. The cycle of reproduction. Journal of Ethnopharmacology. 1992;37(3):179–196. https://doi.org/10.1016/0378-8741(92)90033-N
Sivagama Sundari M, Sornalakshmi V, Tresina PS, Mohan VR. Evaluation of phytochemical screening and in vitro anti-inflammatory activity of mangrove associate plant Pemphis acidula J.R.Forst. & G.Forst. (Lythraceae). International Journal of Health Sciences. 2022;6(S6):10691–10702. https://doi.org/10.53730/ijhs.v6nS6.12879
Masuda T, Iritani K, Yonemori S, Oyama Y, Takeda Y. Isolation and antioxidant activity of galloyl flavonol glycosides from the seashore plant, Pemphis acidula. Bioscience, Biotechnology, and Biochemistry. 2001;65(6):1302–1309. https://doi.org/10.1271/bbb.65.1302
Hardjito L. Antibacterial, antioxidant and topoisomerase-I inhibitor activities of the coastal ethnomedicinal plant Pemphis acidula. Biotropia. 2007;14(2):43–51. https://doi.org/10.11598/btb.2007.14.2.17
Mardianawati N, Sulastri L, Sinaga E. Total phenolics and flavonoids content of Pemphis acidula leaves extract, in vitro antioxidant and antidiabetic activity, and characterization of the extract towards standardized antidiabetic herbal medicine. Jurnal Tumbuhan Obat Indonesia. 2024;17(2):157–169. https://doi.org/10.31002/jtoi.v18i1.1852
Matsuda H, Morikawa T, Toguchida I, Yoshikawa M. Structural requirements of flavonoids and related compounds for aldose reductase inhibitory activity. Chemical and Pharmaceutical Bulletin. 2002;50(6):788–795. https://doi.org/10.1248/cpb.50.788
Idoko A, Emmanuel UEG, Catherine OI. Phytochemical screening of aqueous, ethanol and methanol extracts of Flacourtia indica leaf and ripe fruit. Universal Journal of Pharmaceutical Research. 2022;7(5):18-22. https://doi.org/10.22270/ujpr.v7i5.836
Moonmun D, Majumder R, Lopamudra A. Quantitative phytochemical estimation and evaluation of antioxidant and antibacterial activity of methanol and ethanol extracts of Heliconia rostrata. Indian Journal of Pharmaceutical Sciences. 2017;79(1): 79-90. https://doi.org/10.4172/pharmaceutical-sciences.1000204
Hariutami D, Darmawati S, Permana Atna, Permana A, Zuraida Z. The Effect of Different Solvents on the Content of Black Cumin Seed Extract (Nigella sativa). Jurnal Kefarmasian Indonesia. 2024;14(1):84–97. https://doi.org/10.22435/jki.v14i1.6631
Nadeem HA, Pervaiz M, Ejaz A, Saeed Z, Khan RRM, Younas U. Comparative phytochemical study of methanolic and ethanolic extracts of Thymus linearis and their antibacterial and antioxidant potential. Biomedical Chromatography. 2024;38(3):e5808. https://doi.org/10.1002/bmc.5808
Andzar Fikranus S, Alam T, Nuralih. Uji Aktivitas Sitotoksik Ekstrak Polar, Semipolar, dan Non-Polar Daun Sambiloto (Andrographis paniculata) terhadap Sel Kanker Hati (HepG2). Jurnal Kefarmasian Indonesia. 2022;1:25–30. https://doi.org/10.22435/jki.v0i0.4875
Jomova K, Alomar SY, Valko R, Liska J, Nepovimova E, Kuca K, Valko M. Flavonoids and their role in oxidative stress, inflammation, and human diseases. Chemico-Biological Interactions. 2025;413:111489. https://doi.org/10.1016/j.cbi.2025.111489
Mohan A, Mahadevan GD, Iyer VA, Mukherjee TK, Patel VH, Kumar R, Siddiqui N, Nayak M, Maurya PK, Kumar P. Dietary flavonoids in health and diseases: a concise review of their role in homeostasis and therapeutics. Food Chemistry. 2025;487: 144674. https://doi.org/10.1016/j.foodchem.2025.144674
Zheng X, Zhang X, Zeng F. Biological functions and health benefits of flavonoids in fruits and vegetables: a contemporary review. Foods. 2025;14(2):155. https://doi.org/10.3390/foods14020155
Shim KH, Sharma N, An SSA. Mechanistic insights into the neuroprotective potential of sacred Ficus trees. Nutrients. 2022;14(22):4731. https://doi.org/10.3390/nu14224731
Kuppachi H, Kandasamy V, Balasundaram U. In-silico screening of potential anti-androgenic and anti-oestrogenic phytocompounds from Saraca asoca for polycystic ovary syndrome treatment. Journal of Applied Pharmaceutical Science. 2024;14(2):261–272. http://doi.org/10.7324/JAPS.2024.146675
Huang L, Li Q, Wu J, He Y, Huang J, Xie S, Yang C, Ruan Q, Zhou Z, Deng M. Galangin reduces MPTP-induced dopamine neuron injury via the autophagy dependent-PI3K/AKT pathway. Frontiers in Aging Neuroscience. 2025;17:1568002. https://doi.org/10.3389/fnagi.2025.1568002
Zhang X, Zhong G, Wu H. Exploring the pharmacological mechanisms and therapeutic implications of galangin against neurological disorders. Pharmacological Reports. 2025;77:1217–1231. https://doi.org/10.1007/s43440-025-00756-z
Calabrese EJ, Pressman P, Hayes A, Baldwin L, Agathokleous E, Kapoor H, Dhawan G, Kapoor R, Calabrese V. Kaempferol, a widely ingested dietary flavonoid and supplement, enhances biological performance via hormesis, especially for ageing-related processes. Mechanisms of Ageing and Development. 2025;225:112065. https://doi.org/10.1016/j.mad.2025.112065
Herrera TES, Tello IPS, Mustafa MA, Jamil NY, Alaraj M, Altameem KKA, Alasheqi MQ, Hamoody AH, Alkhafaji AT, Shakir MN, Alshahrani MY. Kaempferol: unveiling its anti-inflammatory properties for therapeutic innovation. Cytokine. 2025;186:157420. https://doi.org/10.1016/j.cyto.2024.156846
Wang Y, Chen C, Li Y, Li R, Wang J, Wu C, Chen H, Shi Y, Wang S, Gao C. Kaempferol inhibits oxidative stress and reduces macrophage pyroptosis by activating the NRF2 signaling pathway. PLoS One. 2025;20(6): e0325189. https://doi.org/10.1371/journal.pone.0325189
Zeng Y, Liu J, Zhang Q, Qin X, Li Z, Sun G, Jin S. The Traditional Uses, Phytochemistry and Pharmacology of Sarcandra glabra (Thunb.) Nakai, a Chinese Herb With Potential for Development: Review. Frontiers in Pharmacology. 2021. 12:652926. https://doi.org/10.3389/fphar.2021.652926
Chen X, Chhun S, Xiang J, Tangjaidee P, Peng Y, Quek SY. Microencapsulation of Cyclocarya paliurus (Batal.) iljinskaja extracts: A promising technique to protect phenolic compounds and antioxidant capacities. Foods. 2021 Dec 1;10(12).
Jakimiuk K. A comprehensive review of robinetin: distribution, biological activity and pharmacokinetic parameters. International Journal of Molecular Sciences. 2025;26(19):9546. https://doi.org/10.3390/ijms26199546
Hayat MF, Rahman AU, Tahir A, Batool M, Ahmed Z, Atique U. Palliative potential of robinetin to avert polystyrene microplastics instigated pulmonary toxicity in rats. Journal of King Saud University – Science. 2024;36(9):103919. https://doi.org/10.1016/j.jksus.2024.103348
Umoh RA, Johnny II, Andy NA, Idio ER, Charles GE, Udoh AE, Udom TA, Owineng DA. Pharmacognostic evaluation of the leaves of Thunbergia laevis Nees (Acanthaceae). Asian Journal of Biochemistry, Genetics and Molecular Biology. 2023;15(3):91–103. https://doi.org/10.9734/ajbgmb/2023/v15i3342
Cheng L, Tengteng J, Ming Z, Bing F. Recent advances in squalene: biological activities, sources, extraction, and delivery systems. Trends in Food Science & Technology. 2024;146:104392. https://doi.org/10.1016/j.tifs.2024.104392
Lin T, Li L, Liang C, Peng L. Network pharmacology-based investigation of the therapeutic mechanisms of action of Danning tablets in nonalcoholic fatty liver disease. Evidence-Based Complementary and Alternative Medicine. 2021;2021: 3495360. https://doi.org/10.1155/2021/3495360
Rajeswaran S, Rajan DK. Neophytadiene: biological activities and drug development prospects. Phytomedicine. 2025;143:156872. https://doi.org/10.1016/j.phymed.2025.156872
Kim DH, Park MH, Choi YJ, Chung KW, Park CH, Jang EJ, An HJ, Yu BP, Chung HY. Molecular study of dietary heptadecane for the anti-inflammatory modulation of NF-κB in the aged kidney. PLoS One. 2013;8(3):e59316. https://doi.org/10.1371/journal.pone.0059316
Sharmin S, Muzahid AA, Islam MM, Yeasmin MS, Dey AK, Uddin MJ, Rana GM, Barmon J, Alam S, Bhuiyan MN, Ahmed NU. Preliminary investigation of GC–MS profiling and antibacterial activities of different solvent extracts from Litchi chinensis Sonn. seed. Scientifica. 2025;2025(1): 7644558. https://doi.org/10.1155/sci5/7644558
Momuat LI, Rompas RM, Pontoh J, Sanger G, Mantiri DMH, Posangi J, Kepei RC. Antioxidant and antibacterial compounds of Gracilaria salicornia from the coastal waters of Nain Island, Indonesia. Aquaculture, Aquarium, Conservation and Legislation. 2024;17(3):885–896.
Akasha R, Moni S, Alshammari MD, Alhaidan EM, Albasher BN, Alrashidi AS, Syed RU. Spectral analysis of bioactive compounds in cold methanolic extracts of commercial broccoli florets: antioxidant analysis and in vitro cytotoxicity in A549 lung cancer cells. Oriental Journal of Chemistry. 2025;41(4):1194–1206. http://dx.doi.org/10.13005/ojc/410415
Muthusamy NK, Duraisamy MD, Anand AK. Gas chromatography–mass spectrometry (GC–MS) analysis of phytoconstituents from leaves of Rhynchoglossum notonianum (Wall.) B.L. Burtt. European Journal of Medicinal Plants. 2025;36(4):7–16.
Toha ZM, Haron NH, Kamal NNSNM, Arsad H. Antioxidant, antiproliferative activities and chemical profile of Clinacanthus nutans leaf extracts processed using two different pre-extraction drying methods. Medicinal & Aromatic Plants. 2020;9(1):1–8. https://doi: 10.35248/2167-0412.20.9.361
Shaaban MT, Ghaly MF, Fahmi SM. Antibacterial activities of hexadecanoic acid methyl ester and green-synthesized silver nanoparticles against multidrug-resistant bacteria. Journal of Basic Microbiology. 2021;61(2): 157-164. https://doi.org/10.1002/jobm.202000665
Gupta V, Tyagi S, Tripathi R. Hexadecanoic acid methyl ester, a potent hepatoprotective compound in leaves of Pistia stratiotes L. The Applied Biology and Chemistry Journal. 2023;4(4):118–120. https://doi.org/10.52679/tabcj.2023.0012
Dias MKHM, Madusanka DMD, Han EJ, Kim MJ, Jeon YJ, Kim HS, Fernando IP, Ahn G. (−)-Loliolide isolated from Sargassum horneri protects against fine dust-induced oxidative stress in human keratinocytes. Antioxidants. 2020;9(6):474. https://doi.org/10.3390/antiox9060474
Silva J, Alves C, Martins A, Susano P, Simões M, Guedes M, Rehfeldt S, Pinteus S, Gaspar H, Rodrigues A, Goettert MI. Loliolide, a new therapeutic option for neurological diseases? In vitro neuroprotective and anti-inflammatory activities of a monoterpenoid lactone isolated from Codium tomentosum. International Journal of Molecular Sciences. 2021;22(4):1815. https://doi.org/10.3390/ijms22041815
Li LL, Zhao HH, Kong CH. (−)-Loliolide, the most ubiquitous lactone, is involved in barnyardgrass-induced rice allelopathy. Journal of Experimental Botany. 2020;71(4): 1449–1458. https://doi.org/10.1093/jxb/erz513
Lee EJ, Lee S, Jang HJ, Yoo W. Loliolide in Sargassum horneri alleviates ultrafine urban particulate matter (PM0.1)-induced inflammation in human RPE cells. International Journal of Molecular Sciences. 2024;25(1):423. https://doi.org/10.3390/ijms25010423
Yang MH, Ha IJ, Ahn J, Kim CK, Lee M, Ahn KS. Potential function of loliolide as a novel blocker of epithelial-mesenchymal transition in colorectal and breast cancer cells. Cellular Signalling. 2023;105: 110610. https://doi.org/10.1016/j.cellsig.2023.110610
Islam MT, Bhuia MS, de Lima JPM, Maia PAA, Ducati BHB, Coutinho DMMH. Phytanic acid, an inconclusive phytol metabolite: a review. Current Research in Toxicology. 2023;5: 100120. https://doi.org/10.1016/j.crtox.2023.100120
Asbaghi O, Sadeghian M, Nazarian B, Sarreshtedari M, Mozaffari-Khosravi H, Maleki V, Alizadeh M, Shokri A, Sadeghi O. The effect of vitamin E supplementation on selected inflammatory biomarkers in adults: a systematic review and meta-analysis of randomized clinical trials. Scientific Reports. 2020;10(1):17234. https://doi.org/10.1038/s41598-020-73741-6
Kaye AD, Thomassen AS, Mashaw SA, MacDonald EM, Waguespack A, Hickey L, Singh A, Gungor D, Kallurkar A, Kaye AM, Shekoohi S. Vitamin E (α-tocopherol): emerging clinical role and adverse risks of supplementation in adults. Cureus. 2025;17(2):e55003. https://doi.org/10.7759/cureus.78679
Chen M, Ghelfi M, Poon JF, Jeon N, Boccalon N, Rubsamen M, Valentino S, Mehta V, Stamper M, Tariq H, Zunica E. Antioxidant-independent activities of alpha-tocopherol. Journal of Biological Chemistry. 2025;301(4):106347. https://doi.org/10.1016/j.jbc.2025.108327
Copyright (c) 2025 Jurnal Kefarmasian Indonesia

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.















