Safety and Efficacy of Dihydroartemisinin-Piperaquine for Intermittent Preventive Treatment of Malaria in Pregnancy: A Systematic Review of Randomized Controlled Trials
Downloads
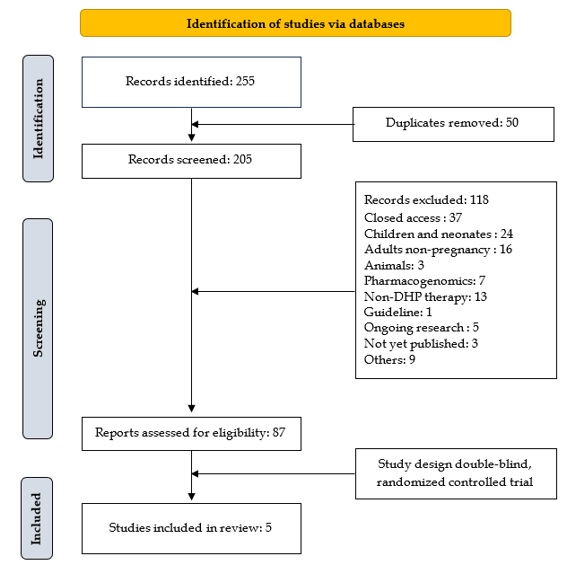
Preventing malaria in pregnant women is crucial, especially for the safety of both the mother and the baby, particularly in malaria-endemic areas. Dihydroartemisinin-Piperaquine (DHP) is one of the options for Intermittent Preventive Treatment in pregnancy (IPTp). Although several previous studies have assessed DHP as a preventive antimalarial in pregnancy, this study systematically consolidates the most recent Randomized Controlled Trials (RCTs), reflecting new evidence and resistance trends to Sulfadoxine-Pyrimethamine (SP) across malaria-endemic regions. This study aims to review the safety and efficacy of DHP use during pregnancy. The methodology involved a comprehensive literature search from the databases PubMed, ScienceDirect, Google Scholar, and Cochrane, published in English from 2020 to 2024. Inclusion criteria encompassed double-blind RCT evaluating the use of DHP during pregnancy. Exclusion criteria included studies that did not involve pregnant women, did not use DHP, and study designs other than double-blind RCT. The initial search yielded 255 articles. After screening for duplicates, a total of 50 duplicates were removed. Ultimately, 5 articles were identified after screening titles, abstracts, and full texts. The analysis results indicate that IPTp DHP is more effective in reducing the incidence of malaria compared to IPTp SP. However, IPTp SP is safer to use than IPTp DHP due to fewer adverse effects. The use of DHP may be considered for IPTp in cases of SP resistance. This review provides an updated synthesis of recent RCTs focusing on the comparative safety and efficacy of DHP versus SP in IPTp of malaria in pregnancy, highlighting recent evidence in the context of emerging SP resistance.
Downloads
Akafity G, Kumi N, Ashong J. Diagnosis and management of malaria in the intensive care unit. Vol. 4, Journal of Intensive Medicine. Chinese Medical Association; 2024. p. 3–15.
Arya A, Kojom Foko LP, Chaudhry S, Sharma A, Singh V. Artemisinin-based combination therapy (ACT) and drug resistance molecular markers: A systematic review of clinical studies from two malaria endemic regions – India and sub-Saharan Africa. Vol. 15, International Journal for Parasitology: Drugs and Drug Resistance. Elsevier Ltd; 2021. p. 43–56.
Varo R, Chaccour C, Bassat Q. Update on malaria. Vol. 155, Medicina Clinica. Ediciones Doyma, S.L.; 2020. p. 395–402.
Septiana E, Bustanussalam B, Rachman F, Hapsari Y, Simanjuntak P. Potensi Ekstrak Kapang Endofit Asal Rimpang Kunyit Sebagai Antimalaria dan Antioksidan. Jurnal Kefarmasian Indonesia. 2017 May 16;7(1).
Emran RK, Hanafi M, Sundowo A, Dewi PN, Adipratiwi N, Ariyani T, et al. Sintesis dan Evaluasi Antimalaria In Vitro Turunan Kinin Terhadap Plasmodium falciparum Synthesis and In Vitro Evaluation of Quinine Derivates Against Plasmodium falciparum. Vol. 2021, Jurnal Kefarmasian Indonesia. Bandung; 2021 Mar. Available from: https://doi.org./10.22
Portal Informasi Indonesia. https://indonesia.go.id/kategori/editorial/8354/pemerintah-targetkan-indonesia-bebas-malaria-pada-2030-strategi-dan-perkembangan?lang=1. 2024. Pemerintah Targetkan Indonesia Bebas Malaria pada 2030: Strategi dan Perkembangan.
Joseph Omang, Antor O Ndep, Dominic Offiong, Fidelis Otu, Kenneth Onyejose. Malaria in Pregnancy in Nigeria: A Literature Review. International Healthcare Research Journal. 2020 Feb 19;3(11):346–8.
Chua CLL, Khoo SKM, Ong JLE, Ramireddi GK, Yeo TW, Teo A. Malaria in Pregnancy: From Placental Infection to Its Abnormal Development and Damage. Vol. 12, Frontiers in Microbiology. Frontiers Media S.A.; 2021.
Al Khaja KAJ, Sequeira RP. Drug treatment and prevention of malaria in pregnancy: a critical review of the guidelines. Vol. 20, Malaria Journal. BioMed Central Ltd; 2021.
Kobia FM, Maiti K, Obimbo MM, Smith R, Gitaka J. Potential pharmacologic interventions targeting TLR signaling in placental malaria. Vol. 38, Trends in Parasitology. Elsevier Ltd; 2022. p. 513–24.
Fernandes S, Were V, Gutman J, Dorsey G, Kakuru A, Desai M, et al. Cost-effectiveness of intermittent preventive treatment with dihydroartemisinin–piperaquine for malaria during pregnancy: an analysis using efficacy results from Uganda and Kenya, and pooled data. Lancet Global Health. 2020 Dec 1;8(12):e1512–23.
Muthoka EN, Usmael K, Embaye SM, Abebe A, Mesfin T, Kazembe D, et al. Safety and tolerability of repeated doses of dihydroartemisinin-piperaquine for intermittent preventive treatment of malaria in pregnancy: a systematic review and an aggregated data meta-analysis of randomized controlled trials. Malaria Journal. 2023 Dec 1;22(1).
Chu X, Li M, Yan P, Feng L, Li J, Liu X, et al. Dihydroartemisinin-piperaquine versus Sulfadoxine-pyrimethamine for malaria during pregnancy: A systematic review and meta-analysis of randomized controlled trials. 2020 May 5: https://doi.org/10.22541/au.158471520.09803558
González R, Nhampossa T, Mombo-Ngoma G, Mischlinger J, Esen M, Tchouatieu AM, et al. Safety and efficacy of dihydroartemisinin–piperaquine for intermittent preventive treatment of malaria in pregnant women with HIV from Gabon and Mozambique: a randomised, double-blind, placebo-controlled trial. Lancet Infectious Disease. 2024 May 1;24(5):476–87.
Poespoprodjo JR, Fobia W, Kenangalem E, Lampah DA, Sugiarto P, Tjitra E, et al. Treatment policy change to dihydroartemisinin-piperaquine contributes to the reduction of adverse maternal and pregnancy outcomes. Malaria Journal. 2015 Jul 15;14(1).
Savic RM, Jagannathan P, Kajubi R, Huang L, Zhang N, Were M, et al. Intermittent Preventive Treatment for Malaria in Pregnancy: Optimization of Target Concentrations of Dihydroartemisinin-Piperaquine. Clinical Infectious Diseases. 2018 Sep 14;67(7):1079–88.
Hoyt J, Hill J, Achieng F, Ouma P, Kariuki S, Desai M, et al. Healthcare provider and pregnant women’s perspectives on the implementation of intermittent screening and treatment with dihydroartemisinin–piperaquine for malaria in pregnancy in western Kenya: a qualitative study. Malaria Journal. 2021 Dec 1;20(1).
Kayiba NK, Yobi DM, Tchakounang VRK, Mvumbi DM, Kabututu PZ, Devleesschauwer B, et al. Evaluation of the usefulness of intermittent preventive treatment of malaria in pregnancy with sulfadoxine-pyrimethamine in a context with increased resistance of Plasmodium falciparum in Kingasani Hospital, Kinshasa in the Democratic Republic of Congo. Infection, Genetics and Evolution. 2021 Oct 1;94.
Cheng K, Aitken EH, Hasang W, Meagher N, Price DJ, Madanitsa M, et al. Intermittent preventive treatment with sulphadoxine-pyrimethamine but not dihydroartemisinin-piperaquine modulates the relationship between inflammatory markers and adverse pregnancy outcomes in Malawi. PLOS Global Public Health. 2024 May 1;4(5).
Health Organization W. WHO Guidelines for malaria - 3 June 2022. 2022.http://apps.who.int/bookorders.
Okoro RN, Geidam AD, Bukar AA, Zarami AB, Ohieku JD, Musa AB, et al. Superiority trial of intermittent treatment with dihydroartemisinin–piperaquine versus sulfadoxine–pyrimethamine for the prevention of malaria during pregnancy. Future Journal of Pharmaceutical Sciences. 2023 Jan 31;9(1).
Dorkenoo AM, Warsame M, Ataba E, Hemou M, Yakpa K, Sossou E, et al. Efficacy of artemether-lumefantrine and dihydroartemisinin-piperaquine and prevalence of molecular markers of anti-malarial drug resistance in children in Togo in 2021. Malaria Journal. 2024 Dec 1;23(1).
Amimo F, Lambert B, Magit A, Sacarlal J, Hashizume M, Shibuya K. Plasmodium falciparum resistance to sulfadoxine-pyrimethamine in Africa: A systematic analysis of national trends. BMJ Global Health. 2020 Nov 19;5(11).
Madanitsa M, Barsosio HC, Minja DTR, Mtove G, Kavishe RA, Dodd J, et al. Effect of monthly intermittent preventive treatment with dihydroartemisinin–piperaquine with and without azithromycin versus monthly sulfadoxine–pyrimethamine on adverse pregnancy outcomes in Africa: a double-blind randomised, partly placebo-controlled trial. The Lancet. 2023 Mar 25;401(10381):1020–36.
Hughes E, Wallender E, Kajubi R, Jagannathan P, Ochieng T, Kakuru A, et al. Piperaquine-Induced QTc Prolongation Decreases With Repeated Monthly Dihydroartemisinin-Piperaquine Dosing in Pregnant Ugandan Women. Clinical Infectious Diseases. 2022 Aug 1;75(3):406–15.
John Lee J, Kakuru A, Jacobson KB, Kamya MR, Kajubi R, Ranjit A, et al. Monthly Sulfadoxine-Pyrimethamine During Pregnancy Prevents Febrile Respiratory Illnesses: A Secondary Analysis of a Malaria Chemoprevention Trial in Uganda. Open Forum Infect Dis. 2024 Apr 1;11(4).
Funck-Brentano C, Bacchieri A, Valentini G, Pace S, Tommasini S, Voiriot P, et al. Effects of Dihydroartemisinin-Piperaquine Phosphate and Artemether-Lumefantrine on QTc Interval Prolongation. Scientific Report. 2019 Dec 1;9(1).
Dela Cruz M, Ershad M, Mostafa A. Qtc interval prolongation associated with inpatient azithromycin therapy for pneumonia. Journal of the American Osteopathic Association. 2021 Jan 1;121(1):5–9.
Kakuru A, Roh ME, Kajubi R, Ochieng T, Ategeka J, Ochokoru H, et al. Infant sex modifies associations between placental malaria and risk of malaria in infancy. Malaria Journal. 2020 Dec 1;19(1).
Mlugu EM, Minzi O, Kamuhabwa AAR, Aklillu E. Effectiveness of Intermittent Preventive Treatment With Dihydroartemisinin-Piperaqunine Against Malaria in Pregnancy in Tanzania: A Randomized Controlled Trial. Clinical Pharmacology & Therapeutics. 2021 Dec 1;110(6):1478–89.
Muthoka EN, Usmael K, Embaye SM, Abebe A, Mesfin T, Kazembe D, et al. Safety and tolerability of repeated doses of dihydroartemisinin-piperaquine for intermittent preventive treatment of malaria in pregnancy: a systematic review and an aggregated data meta-analysis of randomized controlled trials. Malaria Journal. 2023 Dec 1;22(1).
Copyright (c) 2025 Jurnal Kefarmasian Indonesia

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.















